Telematic support for collaborative doctors networks in clinical research
H.-D. Stahl, M. Egbring, H. EnglischInt. J. Pharmaceutical Med. 2000, 14(6): 329-331
Abstract
We previously reported on the setup of collaborative networks of doctors in private practice to jointly conduct clinical studies (1). These networks presently encompass more than 500 doctors the largest group being General Practitioners (GPs, > 400 members). We report here on a pilot project in the context of a phase II study to provide telematic support to a group of about 10 private doctors to achieve 2 main objectives: firstly, to set up a patient registry of an epidemiologically important disease, in this case Chronic Obstructive Pulmonary Disease (COPD). Secondly, to further improve the quality of clinical study conduct especially with regard to source data verification.Introduction
One of the major obstacles for international clinical research is reliable recruitment of large number of patients per investigational site. Registration authorities and payor institutions are increasingly asking for long-term epidemiological data i.e. impact of novel therapies on mortality, survival and work capacity. Numerous novel compounds generated by preclinical research are expected to reach the developmental stage of clinical testing (Phase II - III) in the near future and may further aggravate the problem.One approach to meet the new challenges in Germany is the inclusion of the private doctor`s sector to improve recruitment of suitable patients. These patients are referred to a central study site ("Studienambulanz") where the clinical trial is conducted according to ICH-GCP guidelines (1). Since the investigators working at the central study site see the trial patients at intervals determined by the study protocol, an exchange of information with the doctor caring for the patient in daily clinical life is essential. Therefore, one of the major goals of the collaborative networks in Leipzig is to electronically link private doctors and central study site in such a way that highly sensitive data can be exchanged under strict adherence to the highest safety standards (2). We report here on a pilot project in the context of a phase II study testing a novel phosphodiesterase inhibitor in COPD. We established an intranet including 10 private doctors to facilitate a patient registry and source data verification according to ICH-GCP.
Starting point
One has to realize that most doctors in East Germany commenced private practice after 1990. Approximately 70% use computers for patient data management. However, one serious problem lies in the fact that over 100 different software systems are currently employed by private doctors. All of them have in common an interface which facilitates the transfer of data to the association of private doctors called "Kassenärztliche Vereinigung" (KV). This interface is vital since the transfer of data is a prerequisite for the remuneration of private doctors. In addition, many software systems provide interfaces for laboratory results, which can be used also for receiving non-standardized laboratory reports (text files). It is important to establish a close and trusting relationship with software specialist liased with the private doctors. We opted to implement the pilot project with 3 software systems often used amongst GPs to start with. Since data security is of upmost importance for private doctors we included the IT consulting company SYSECA GmbH (www.thales-is.com).The Telematic Project and the Flow of Information
The data exchange is facilitated between different sites with the central study site being at the centre (Fig. 1).A. Registry Data from the Private Doctor to the central study site
The patients in the anonymous registry "COPD" can be identified only by a number known to the private doctor.The registry contains data such as the FEV1 before and after taking Salbutamol, history of smoking and concomitant diagnosis. Since the phase II study COPD specified a total of 38 in- and exclusion criteria, it was unrealistic to expect a private doctor to consider all of them when he is consulted by a patient. Furthermore, for studies with a small time window for recruitment the probability is high that candidates for the study are not consulting the doctor during the recruitment phase. In our case e.g. one patient visited the central study site who had not seen his pulmologist for 2 years.
Thus, about 1000 patients were included in the registry to ensure that at least 50 of them may fulfill all 38 criteria. The investigator in the central study site checked the criteria for all patients and asked the private doctor to invite suitable patients to visit the central study site for prescreening. This applied to about one third of the patients. After 5 months, 100 patients visited our central study site for prescreening. The main reason for exclusion of patients after the prescreening visit was concomitant medication.
The registry was started one month prior to the ethics committee of the University of Leipzig deciding on the study. Thus, the first patients visited the central study site for prescreening the day following the ethics committee's approval.
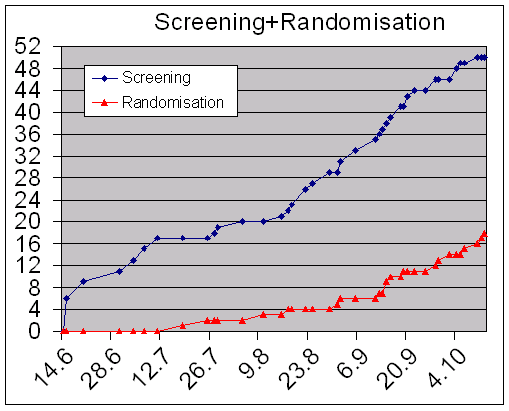
Fig. 2 demonstrates screening and randomization of patients for the phase II study. 4 months following the initiation visit the ZET ranked in the top 2 of 19 centers in Germany.
B. Source Data from the Private Doctor to the central study site
This concerns source data such as medical history, results of previous investigations, adverse events (AEs) and current treatment. On entering new clinical data the doctor is asked whether this information should be forwarded to the server of the central study site. This is classified as a source data document and checked by monitors and auditors at the central study site. In addition an electronic or paper copy will be kept in the private doctor's patient file in his rooms.C. From the central study site to the Private Doctor
Results of investigations generated in the context of the clinical trial are transfered to the private doctor. This is helpful in the daily clinical care for the patient. Furthermore, important information for example on randomisation of the patient can be quickly forwarded to the doctor.D. From the central study site to the Sponsor
Data from patient`s visits including AEs and results of investigations are transfered to the sponsor within 24 hours. This was done in the current phase II study in a classical RDE system (4).E. From the Sponsor to the central study site
Results of investigations, discrepancy reports and other important information regarding the clinical trial are forwarded to the central study site and subsequently - if necessary - relayed to the GPs. Due to lack of time for programming interfaces up to now there is no connection between the established RDE system of the sponsor and the pilot system to the private doctors. Hence, the forwarding procedure includes manual work.F. From the central study site to the Public
We anticipate that Patients with uncontrolled disease and their relatives will increasingly and systematically search in the Internet for novel therapeutic options as offered in the context of Clinical Trials. The homepage of the ZET e.V. (www.zet-ev.de, in the future www.therapiestudien.de) aims at informing patients on current clinical studies and their respective indications.Summary and Outlook
In this paper we report on a pilot project providing telematic support to collaborative doctors´ network in clinical research. An intranet connecting 10 private physicians and the central study site proved to be helpful in a more efficient recruitment of patients and higher quality of study conduct, i.e. source data verification. Indeed, the telematic support for 2 respiratory physicians to establish the patient registry proved more efficient in terms of recruitment than the 5 other respiratory physicians applying the standard way of recruitment. Some physicians considered the participation in the intranet an additional motivation for participation in the study.The main reason for exclusion of patients in Leipzig from the trial in COPD during presreening was concomitant medication. Therefore, concomitant medication should be included in future registries.
The reason for exclusion during screening was mainly due to too high FEV1 values after Salbutamol obtained by the investigators at the central study site. This may reflect uneven technical standards with regard to respiration function tests or varying collaboration of patients.
The patient registry of the ZET e.V. will be built up step by step to contain the epidemiologically most important diseases, i.e. rheumatoid arthritis, diabetes, osteoarthritis, hypertension, asthma and cardiac failure.
Depending on the clinical study proposed by international sponsors further disease entities will be introduced into the patient registry.
We anticipate significant advances with regard to reliable planning of clinical studies, more efficient recruitment of patients and quality of conduct of clinical trials in the context of collaborative doctor networks. Overall, this will lead to earlier marketing applications and cost savings.
Furthermore, if the telematic support can be extended to all our collaborating physicians (>500 currently) studies in the area of health economics will be feasible. We foresee increasing pressure from health care providers such as insurance companies to generate data on the impact of costly novel therapies on mortality, survival and especially work capacity.
Advantages on market introduction of a novel compound are self-evident from a marketing point-of-view.
Acknowledgement
We like to thank Drs. Wingen, Rappard and Pröve (Bayer Vital GmbH) for helpful discussions.References
| 1 | H.-D. Stahl and F. Emmrich, Collaborative Networks with Private Doctors to conduct joint Clinical Studies in Leipzig/Germany. Int.J.Pharm.Med. 2000; 14; 27-28. |
| 2 | A von Heydwolff, http://ourworld.compuserve.com/homepages/gesundheitsdatenschutz. |
| 3 | Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al., The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31; 315-24. |
| 4 | J. Pröve, "Neuer Anlauf vor drei Jahren", Arzneimittelzeitung Spezial, 11.11.1999. |
Fig 1.

Fig 2.